Multiscale modelling of transepithelial fluid flows
We are developing multiscale models of the regulatory
processes
controlling transepithelial fluid flow in saliva secretion in the
parotid gland (the largest of the salivary glands) and in lung
epithelium, which regulates the depth of the fluid layer bathing the
airways of the respiratory system.
|
|
The
decreased ability to produce adequate levels of saliva has been
associated with numerous subjective and objective functional deficits,
including the sensation of oral dryness (xerostomia), difficulty with
speaking, mastication and swallowing, and an increased susceptibility
to caries development and opportunistic infections (e.g., Candida
albicans). Treatments are often only partially effective, frequently
producing adverse side-effects and usually requiring life-long use. An
important step in improving these treatments is a thorough
understanding of the molecular pathways involved in saliva secretion.
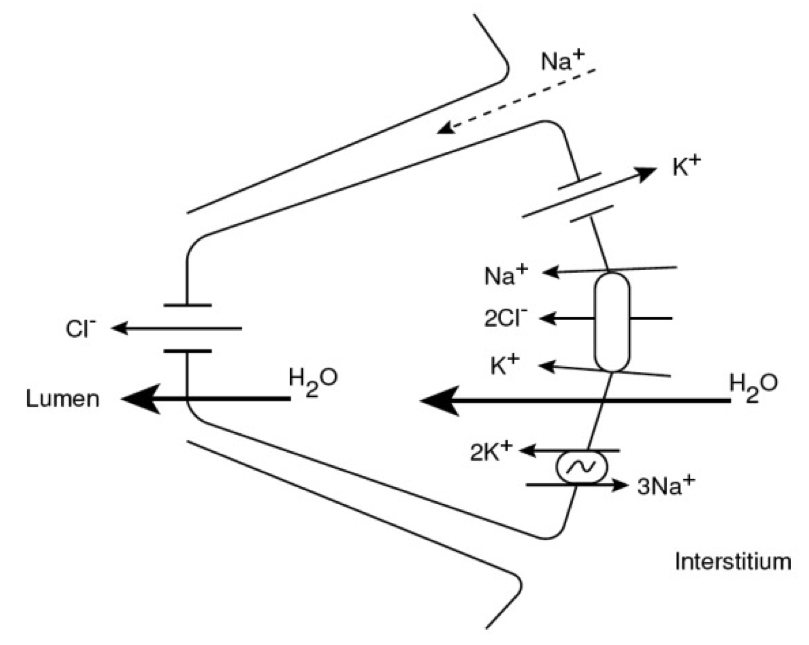
We are constructing a multiscale mathematical model of a prototypical
salivary gland secretory unit, an acinus and attached duct, spanning
from molecular to tissue level properties, in order to simulate human
physiological fluid secretion and to
test the role of several molecular and cellular elements in the
secretion process. |
The
airways of the lung present an interface between the external
environment and the systemic circulation. Exposure to atmospheric gases
provides the source of oxygen, required for respiration, but also
brings the possibility of inspiration of toxic or otherwise pernicious
materials. The mucociliary transport system in the airways provides the
front-line defense against inspired toxins. Without adequate hydration
of the thin layer of liquid lining the airways, mucociliary transport
would cease, leading to build up of mucus and impairing the clearance
of any inhaled debris. Dysfunction of the mucociliary clearance system
has detrimental pathological consequences, for example, the genetic
disease Cystic Fibrosis. We are developing a multi-scale computational
model of airway epithelial fluid transport to investigate the transport
of water and heat within the airways, with which to assess current
clinical metrics used to prescribe inspired air humidity for
artificially ventilated patients. |
|
Key Publications:
L.
Palk, J. Sneyd, T.J. Shuttleworth, D.I. Yule, E.J. Crampin
A dynamic
model of saliva secretion
Journal of Theoretical Biology
2010 (doi:10.1016/j.jtbi.2010.06.027)
N.J. Warren, E.J. Crampin, M.H. Tawhai
The role of
airway epithelium in replenishment of evaporated airway surface liquid
from the human conducting airways
Annals of Biomedical
Engineering 2010 (doi:10.1007/s10439-010-0111-6)
N.
Warren, M.H. Tawhai, E.J. Crampin
The effect
of intracellular calcium oscillations on fluid secretion in airway
epithelium
Journal of Theoretical Biology 265,
270-277, 2010
N.J.
Warren, M.H. Tawhai, E.J. Crampin
Mathematical
modelling of calcium wave propagation in airway epithelium: Evidence
for regenerative ATP release
Experimental
Physiology
95, 232-249, 2010
N.J.
Warren, M.H. Tawhai, E.J. Crampin
A
Mathematical Model of Calcium-Induced Fluid Secretion in Airway
Epithelium
Journal of
Theoretical Biology 259 (4), 837-849, 2009
E. Gin,
E.J.
Crampin, D.A. Brown, T.J. Shuttleworth, D.I. Yule,
J. Sneyd
A
mathematical model of fluid secretion from a parotid acinar cell
Journal of
Theoretical Biology 248,
64-80, 2007
Collaborations:
Merryn Tawhai, ABI
James
Sneyd, Department
of Mathematics, University of Auckland
Ted Begenisich, Jim
Melvin, David Yule and Trevor Shuttleworth groups, University of
Rochester
Recent Funding:
|