Virtual heart disease: signalling, metabolism and
mitochondrial dysfunction in heart disease
We use mathematical modelling to examine cellular mechanisms
underlying cardiac disease.
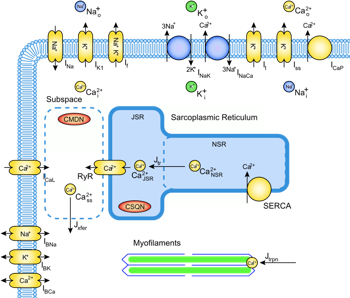 Cardiac
ischaemia arises when coronary arteries on the surface of the
heart become narrow or blocked. The resulting reduction of blood
flow
deprives a region of the heart muscle of oxygen delivery,
leading to significant changes to the normal function of individual
heart cells. The condition is often fatal. The effects of ischaemia on
heart muscle are complex and multifactorial,
involving changes to energy metabolism, pH regulation, ionic
homeostasis
and electrophysiology which combine to produce the clinical symptoms of
the
disease. Integrative modelling provides a means of assessing the
quantitative contribution of each of these components, and assessing
potential therapeutic strategies. We are developing models of cardiac
bioenergetics coupled to mitochondrial function, electrophysiology
and excitation- contraction coupling to investigate the development of
pathology, and applying these models to aspects of ischaemic heart
disease including acidosis, hyperkalaemia, and mitochondrial
dysfunction. Cardiac
ischaemia arises when coronary arteries on the surface of the
heart become narrow or blocked. The resulting reduction of blood
flow
deprives a region of the heart muscle of oxygen delivery,
leading to significant changes to the normal function of individual
heart cells. The condition is often fatal. The effects of ischaemia on
heart muscle are complex and multifactorial,
involving changes to energy metabolism, pH regulation, ionic
homeostasis
and electrophysiology which combine to produce the clinical symptoms of
the
disease. Integrative modelling provides a means of assessing the
quantitative contribution of each of these components, and assessing
potential therapeutic strategies. We are developing models of cardiac
bioenergetics coupled to mitochondrial function, electrophysiology
and excitation- contraction coupling to investigate the development of
pathology, and applying these models to aspects of ischaemic heart
disease including acidosis, hyperkalaemia, and mitochondrial
dysfunction.
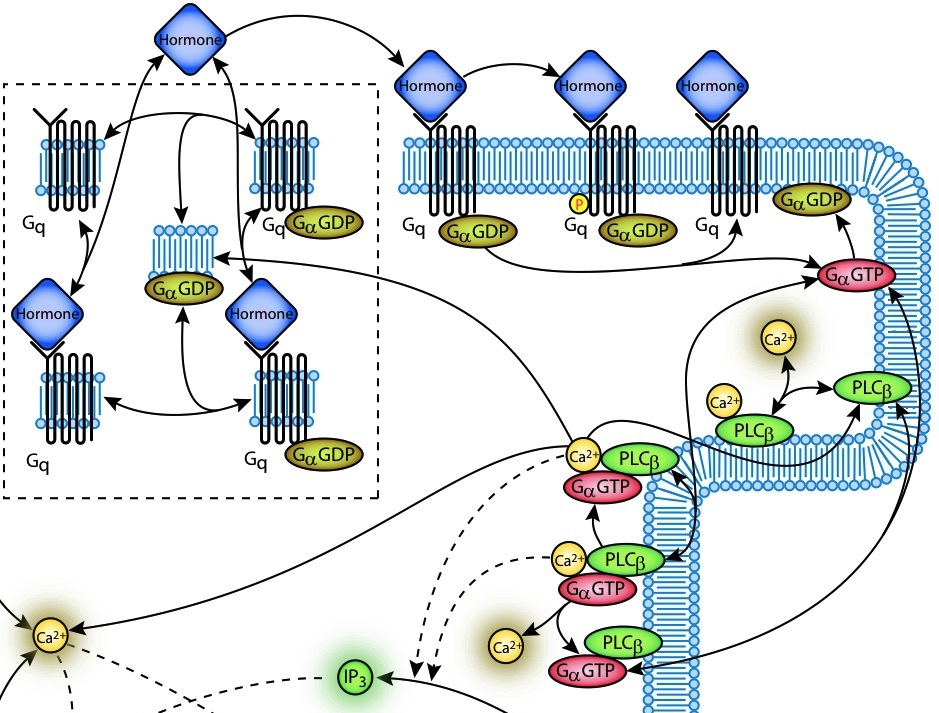
Signal
transduction pathways regulate all aspects of cardiac cell physiology:
detecting, amplifying, and integrating external stimuli to trigger
intracellular responses such as changes in gene expression, or to
modify enzyme or ion channel activity. We are developing models
of
key signalling pathways involved in regulating excitation-contraction
coupling in the heart, focusing those signalling pathways thought to be
responsible for mediating heart disease, in
particular hypertrophy in the failing heart.
Key Publications:
J.-C. Han, A.J. Taberner, K. Tran, S. Goo, D.P.
Nickerson, M.P. Nash, P.M.F. Nielsen, E.J. Crampin, D.S
Loiselle
Comparison of the Gibbs and Suga Formulations of Cardiac
Energetics: the Demise of 'Isoefficiency'
Journal of Applied
Physiology, doi:10.1152/japplphysiol.00693.2011
M.
Fink, S.A. Niederer, E.M. Cherry, F.H. Fenton, J.T. Koivumaki, G.
Seemann, R. Thul, H. Zhang, F.B. Sachse*, D.A. Beard*, E.J. Crampin*, N.P.
Smith*
Cardiac
cell modelling: Observations from the heart of the cardiac physiome
project
Progress in Biophysics
& Molecular Biology 104 (1-3), 2-21, 2011
D. Loiselle
D., K. Tran, E.J. Crampin, N. Curtin.
Why
has Reversal of the Actin-Myosin Cross-Bridge Cycle Not Been Observed
Experimentally?
Journal of Applied Physiology
108, 1465-1471, 2010
K. Tran,
N.P. Smith, D.S. Loiselle, E.J. Crampin
A
metabolite-sensitive, thermodynamically-constrained model of cardiac
cross-bridge cycling: Implications for force development during ischemia
Biophysical
Journal 98, 267-276, 2010
K.
Tran, N.P. Smith, D.S. Loiselle, E.J. Crampin
A
thermodynamic model of the cardiac sarcoplasmic/endoplasmic Ca2+
(SERCA) pump
Biophysical Journal
96
(5), 2029-2042, 2009
M.T. Cooling, P.J.
Hunter, E.J. Crampin
Sensitivity of NFAT
cycling to cytosolic calcium: Implications for hypertrophic signalling
in cardiac myocytes
Biophysical Journal 96
(6), 2095-2104, 2009
J.
Terkildsen, E.J. Crampin, N.P. Smith
The
balance between inactivation and activation of the Na+-K+ pump
underlies the triphasic accumulation of extracellular K+ ions during
myocardial ischemia
American Journal
of Physiology, Heart and Circulatory Physiology 293,
H3036-H3045,
2007
M.
Cooling, P.J. Hunter, E.J. Crampin
Modelling
hypertrophic IP3 transients in the cardiac myocyte
Biophysical Journal 93
3421-3433, 2007
E.J. Crampin,
N.P. Smith
A
dynamic
model of excitation-contraction coupling during acidosis in cardiac
ventricular myocytes
Biophysical Journal 90,
3074-3090, 2006
N.P. Smith, E.J.
Crampin
Development
of
models of active ion transport for whole-cell modelling: Cardiac
sodium-potassium pump as a case study
Progress in Biophysics & Molecular Biology
85 (2-3),
387-405, 2004
E.J. Crampin, M.
Halstead, P. Hunter, P. Nielsen, D. Noble, N.
Smith, M. Tawhai
Computational
Physiology and the Physiome Project
Experimental Physiology 89
(1), 1-26, 2004
International Collaborations:
Dan Beard (Medical College Wisconsin),
Nic Smith (University of Oxford)
Recent Funding:
|

